GENERAL CHEMISTRY TOPICS
Acids and bases - An introduction
Acids and bases: a definition The common acids and bases, strong and weak pH, pOH, and other logarithmic functions
Acids and bases are familiar to most of us, manly in terms of their general qualitative features, but a chemist seeks to understand them on a more fundamental level. An acquaintance with the chemistry of ions in solution serves as a good initial starting point for the study of acid-base chemistry, since acids and bases in aqueous solution (where water is the solvent) can be viewed as the presence of specific types of ions. The first and simplest definition of an acid is the Arrhenius definition, which states that an acid is a substance that creates or consists of hydrogen ions in water, H+(aq). The corresponding Arrhenius definition of a base is a substance that creates or consists of hydroxide ions in water, OH–(aq).
There are seven common strong acids (listed in the table below) that ionize completely to hydronium ion and a conjugate anion in water. This makes strong acids strong electrolytes. Other acids only partially ionize - these are the weak acids, which correspondingly are weak electrolytes. A primary example of a weak acid is the common organic acid acetic acid (ethanoic acid). Vinegar contains acetic acid at a low concentration. The formula for acetic acid is typically written as CH3COOH, with the acidic hydrogen listed last. Note that all the acids listed in the table are molecular compounds. Examples of strong bases are the soluble metal hydroxides, the most common being sodium hydroxide (NaOH) and potassium hydroxide (KOH). Our primary example of a weak molecular base is aqueous ammonia, NH3(aq). In aqueous solution, a small proportion of the aquated ammonia molecules react to form aqueous ammonium and hydroxide ions.
Acids and bases have long been known to be chemical opposites, as they undergo neutralization reactions. An example of a neutralization reaction is considered below. An acid-base titration is a quantitative, stoichiometric measurement of a neutralization reaction.
Strong acids |
Weak acids | |||||||
| acid name and formula | type | acid name and formula | type | |||||
| hydrochloric acid - HCl hydrobromic acid - HBr hydroiodic acid - HI |
hydrogen halides | hydrofluoric acid - HF | hydrogen halide | |||||
| phosphoric acid - H3PO4 sulfurous acid - H2SO3 chlorous acid - HClO2 hypochlorous acid - HClO carbonic acid - H2CO3 |
oxyacids | |||||||
| nitric acid - HNO3 sulfuric acid - H2SO4 perchloric acid - HClO4 chloric acid - HClO3 |
oxyacids | |||||||
| see special note below* | ||||||||
| formic acid - HCOOH acetic acid - CH3COOH benzoic acid - C6H5COOH |
organic (carboxylic acids) | |||||||
*Special note on carbonic acid: H2CO3 decomposes to carbon dioxide and water:
Carbon dioxide has limited solubility in water under atmospheric pressure, so the characteristic fizz of carbonated water is observed:
|
||||||||
Strong bases |
Weak bases | |||||||
| base name and formula | type | base name and formula | type | |||||
| lithium hydroxide - LiOH sodium hydroxide - NaOH potassium hydroxide - KOH rubidium hydroxide - RbOH cesium hydroxide - CsOH |
alkali metal hydroxide | ammonia - NH3 | molecular | |||||
| carbonate - CO32–(aq) | oxoanions | |||||||
| tetraalkylammonium hydroxide, R4NOH # | quaternary ammonium hydroxide | |||||||
| methylamine - CH3NH2 |
organic (amines, based on ammonia) | |||||||
| sodium oxide - Na2O potassium oxide - K2O |
metal oxides (base anhydrides) | |||||||
| acetate - CH3COO– | organic anions (e.g. carboxylates) | |||||||
|
# Note: Like the metal hydroxides, these are compounds with ionic character. They are composed of the quaternary amine cation, tetraalkylammonium (R is an alkyl group such as methyl, CH3), and the hydroxide anion: [R4N+] and OH–, respectively. Some sources consider insoluble hydroxides such as Mg(OH)2 to be weak bases. They create relatively little OH–(aq) because of their low solubility. |
||||||||
pH, pOH, and other logarithmic functions
In order to determine not only whether a given compound acts as an acid or base when mixed with water, but also to measure how acidic or how basic a given aqueous solution may be, the pH scale is defined. The pH is a logarithmic function of the H+(aq) concentration,
pH = − log [H+]
Note the minus sign in the definition creates an inverse relationship between [H+] and pH. A very high [H+] means pH is low, and vice-versa. But pH varies much less than [H+] in magnitude. This is characteristic of logarithmic functions, and make them useful in cases where quantities vary over many orders of magnitude. Another example of such a quantity is Keq, the equilibrium constant equilibrium constant for a reaction. We will want to be able to use pK values, where
pK = − log K
to quantify the strength of acids on the same scale as the pH scale.
In the range of hydronium ion concentrations we will typically encounter, the pH ranges between 0 and 14. As we may well know already, a pH of 7 - that is, [H+] = 1.0 × 10−7 M - is considered neutral, and pH less than 7 is considered acidic, while pH > 7 is basic. As a measure of basicity, and a counterpart to pH, we introduce pOH, defined as
pOH = − log [ OH − ]
There is a simple but important relationship between pH and pOH in aqueous solutions of acidic and basic compounds
pH + pOH = 14
which has its basis in the definition and value of the ionization constant Kw, which is discussed further here.
Acid-base reactions
A variety of reactions involving acids and bases can be described. A strong acid readily reacts with a strong base in what is called a neutralization reaction. Other examples include the reaction of a strong acid and carbonate-containing solutions, and the reaction of carbon dioxide (an example of an acid anhydride) with water.
Acid-base neutralization
Our primary example of a strong acid is hydrochloric acid, HCl(aq). Hydrochloric acid forms from the dissolution of hydrogen chloride, a heterodiatomic gas, into water:
HCl(g) → HCl(aq)
As a strong acid, hydrochloric acid is a strong electrolyte, since the hydrogen chloride molecule in water reacts completely with water to create ions. This is represented by the equation
HCl(aq) + H2O(l) → H3O+(aq) + Cl−(aq)
or in an equivalent, often-used condensed form
HCl(aq) → H+(aq) + Cl−(aq)
Like the dissolution of any water-soluble ionic compound (e.g. sodium chloride), HCl converts completely to ions in water. Soluble ionic compounds and strong acids are both strong electrolytes, yet the latter are molecular compounds that actually react with water, as indicated by the production of hydronium ion, or H3O+(aq), which is commonly abbreviated as H+(aq), as shown in the second equation above. The strong base sodium hydroxide (NaOH) is an ionic compound that is highly soluble in water and completely dissociates into its component ions:
NaOH(aq) → Na+(aq) + OH−(aq)
The mixing together of solutions of hydrochloric acid and sodium hydroxide results in an acid-base neutralization reaction. A series of chemical equations below illustrate the several forms reactions involving ionic species can be represented.
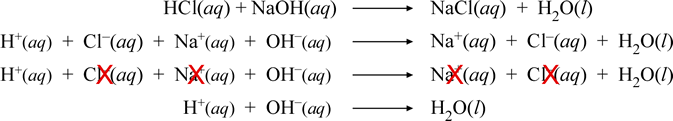
Let's look at this series, each equation in turn. The first equation is the so-called "molecular" or neutral formula equation. The second equation is the complete ionic equation. Next, the full ionic equation is repeated, but cancellation of species appearing the same on both sides is indicated, which leads to the final equation, the net ionic equation. For the neutralization reaction between any monoprotic strong acid and strong base, the resulting net ionic equation will be the same as that shown above for HCl and NaOH.
As another example, consider the reaction occurring between strong acid and carbonate compounds. If hydrochloric acid and sodium carbonate solutions are mixed, what products would we predict for a reaction?
HCl(aq) + Na2CO3(aq) → ?
Here in switching ionic partners, hydrogen plays the role of the cation to be paired with carbonate, while sodium and chloride are a soluble pairing of ions. The first equation shows the products predicted to be formed, the equation balanced with all species represented as neutral formula units (the "molecular" equation). The equation is correct, however in order to account for what is actually observed in this case, the chemistry of the product H2CO3, known as carbonic acid, must be incorporated.
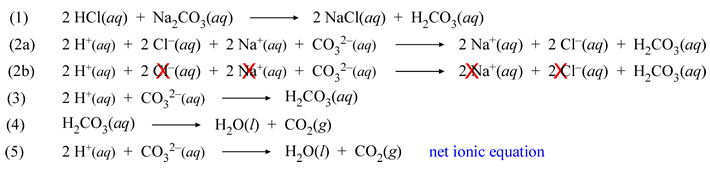
Nonetheless, the ionic forms of this first equation can be written. Equation (2a) shows the complete ionic equation, and (2b) the same, but with the spectator ions that will drop out to yield the net ionic equation marked with crosses. The net ionic equation (3) shows the production of carbonic acid from hydrogen (hydronium) and carbonate ions. As demonstrated in the lab (in which a scheme for identification of an anion in solution, based on solubility and other observations, is developed) the mixing of hydrochloric acid and sodium carbonate results in vigorous bubbling, indicating production of a gas. The instability of carbonic acid accounts for this observation. Its propensity to decompose to water and carbon dioxide is represented by equation (4). The sum of equations (3) and (4) yields equation (5), which is the true net ionic equation for the complete reaction observed for strong acid and carbonate.
The above example shows the production of CO2 driven by the combination of strong acid and carbonate ions. What happens when carbon dioxide dissolves in water, as in the production of carbonated beverages, or as global atmospheric CO2 levels rise? The first reaction would be the reverse of (4) above, i.e. the reaction of carbon dioxide with water to produce carbonic acid. The latter can dissociate (rather than decompose back to reactants) to produce hydrogen (hydronium) ion in water:
H2CO3(aq) → H+(aq) + HCO3−(aq)
What this tells us is that carbon dioxide has some tendency to react with water (in what we can refer to as the "soda pop reaction"), and that when CO2 is abundant, this reaction acidifies the water. Carbon dioxide and some other nonmetal oxides such as SO2 and SO3 are known as acid anhydrides for their ability to acidify water.
A counterpart to the reaction of carbon dioxide with strong acid is the reaction of strong base with ammonium ion, leading to the production of water and ammonia. The sequence of equations below again shows the progression from a molecular/neutral formula equation to the final net ionic equation via the complete ionic equation from which spectator ions get cancelled out.
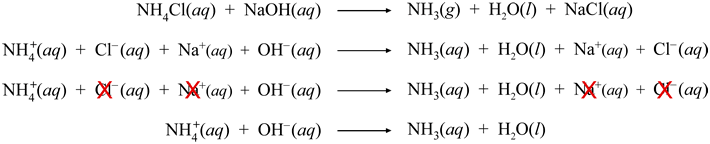
Some of the ammonia produced in this reaction leaves aqueous phase as pure, gaseous ammonia, NH3(g). Its odor is apparent when this reaction is carried out in the laboratory. Note that the formal product NH4OH predicted from the reactants in the first equation by exchanging ionic partners is best thought of as an intermediate leading to the stable products ammonia and water.
Further exploration of acid-base chemistry
A broader framework for acids, bases, and their reactions is provided by the Brønsted-Lowry definition. Weak acids and bases provide important examples of chemical equilibria, and an understanding of weak acid and weak base equilibria is indispensable to biological applications of general chemistry.