Lecture 20. Biochemical signaling
Tuesday 9 April 2019
Selected topics in signal transduction. Hormones. Pancreatic peptide hormones, steroids. Heterotrimeric G proteins. Adenylate cyclase, cyclic AMP, and protein kinase A.
Reading: Lehninger - Ch.12, pp.437-461.
Summary
Reading summary.
§12.1 General features of signal transduction.
§12.2 G protein-coupled receptors and second messengers.
β-adrenergic receptor system acts through the second messenger cAMP.
Box 12-1 G proteins: Binary switches in health and disease (pp.444-446t).
Table 12-2: Some enzymes and other proteins regulated by cAMP-dependent phosphorylation (by PKA).
Several mechanisms cause termination of the β-adrenergic response.
The β-adrenergic receptor is desensitized by phosphorylation and by association with arrestin.
Cyclic AMP acts as a second messenger for many regulatory molecules.
Diacylglycerol, inositol triphosphate, and Ca2+ have related roles as second messengers.
Box 12-2 -Methods- FRET: Biochemistry visualized in a living cell (pp.452-453).
Calcium is a second messenger that is localized in space and time.
Table 12-5: Some proteins regulated by Ca2+ and calmodulin (p.455).
§12.3 GPCRs in vision, olfaction, and gustation.
The vertebrate eye uses classic GPCR mechanisms.
Box 12-3 -Medicine- Color blindness: John Dalton's experiment from the grave (p.455).
Vertebrate olfaction and gustation use mechanisms similar to the visual system.
All GPCR systems share universal features.
Table 12-6: Some signals that act through GPCRs (p.459).
***
Hormones
Hormones are molecules that act as messengers in a living organism, causing specific responses in target cells or tissues. Typically, hormones are polypeptides, steroids, or derivatives of amino acids. The effects and actions of hormones can be long-range and fairly long-lived - such is the case for endocrine hormones such as epinephrine, glucagon, and insulin. Paracrine hormones have a more localized action, and autocrine hormones are those whose action is limited to the cells that produced them.
Epinephrine
A member of the catecholamine class of neurotransmitters, epinephrine (also known as adrenaline) is formed from tyrosine, via dopamine and norepinephrine - two other catecholamine neurotransmitters.
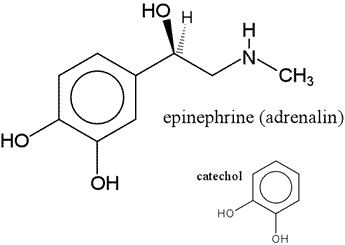
Epinephrine is formed in the adrenal medulla by the action of phenylethanolamine-N-methyltransferase on norepinephrine. The reaction uses S-adenosylmethionine as the methyl group donor. Epinephrine acts as a hormone by traveling through the bloodstream and acting on a variety of target tissues. One its principal effects is the stimulation of the breakdown of glycogen stored in skeletal muscle (and to a lesser degree, that in liver, which is more responsive to glucagon). Epinephrine binds to the extracellular portion of a plasma membrane receptor, triggering a cascade of signaling events, one of the hallmarks of which is increased intracellular levels of cyclic AMP, a so-called "second messenger". In other words, the signal delivered by the "first messenger", i.e. the hormone is transduced into an intracellular signal. The cAMP molecules act like an intracellular hormone, able to diffuse throughout the cytoplasm and exerting further downstream effects. In what follows, this signal transduction pathway is more fully delineated.
G proteins
G proteins are a large class of signaling proteins that are activated by binding guanosine triphosphate (GTP), and are deactivated as a result of an intrinsic GTPase activity. There are actually two classes of G proteins: heterotrimeric G proteins, consisting of α, β, and γ (alpha, beta, and gamma) subunits that are coupled to receptor proteins that are integral membrane proteins with the architecture of seven transmembrane α helices - these are commonly denoted G protein coupled receptors (GPCRs); and small G proteins, monomeric members of the Ras superfamily.
As a paradigm of the signal transduction mediated by heterotrimeric G proteins coupled to GPCRs, the case of the pancreatic hormone glucagon binding to glucagon receptors exposed on the surface of hepatocytes (liver cells) serves well. When glucagon binds to the GPCR glucagon receptor, a conformational change occurs in the latter that affects its interaction with the Gα subunit of its associated heterotrimeric G protein, in turn inducing the Gα subunit to exchange its bound GDP molecule for GTP. This activation entails the dissociation of the Gα subunit from the Gβγ subunit; these dissociated parts of the heterotrimer have separate downstream signaling effects. The Gα subunit, in this case denoted Gsα, stimulates the activity of another membrane-associated protein, adenylate cyclase. This results in the intracellular production of 3′,5′-cyclic AMP (cAMP), a so-called "second messenger" that signals further downstream effects such as a protein kinase cascade that stimulates the liver to produce more glucose for export to the bloodstream by turning on within hepatocytes the activity of glycogen phosphorylase via its phosphorylation
Protein kinase A (PKA)
A kinase is an enzyme that transfers a phosphate group - usually from the γ-phosphate from ATP - to a substrate molecule. If the substrate is a protein, the enzyme is referred to as a protein kinase. Kinases are ubiquitous metabolic and regulatory enzymes, and mediate links of signal transduction pathways. A particularly important and instructive example of the latter is protein kinase A (PKA, or cAMP-dependent protein kinase, cAPK) Once specifically activated by cAMP, PKA [EC 2.7.11.11] catalyzes the phosphorylation of a variety of intracellular targets, including the bifunctional phosphofructokinase/fructose 2,6-bisphosphatase, phosphorylase kinase, glycogen synthase, hormone-sensitive lipase, and acetyl CoA carboxylase.
The kinase is a heterotetramer of two catalytic and two regulatory subunits. The binding of cAMP to regulatory subunits triggers the dissociation of the complex, liberating free catalytic subunits.
C(R2)C (inactive) + 4 cAMP → 2 C (active) + (R·2 cAMP)2
The reaction the free, active catalytic subunit of PKA catalyzes is
ATP + protein = ADP + phosphoprotein
Protein kinase A is a key component of a signal transduction pathway that is mediated by cAMP. The latter is often termed a second messenger, in that it acts intracellularly to transmit an extracellular signal (such as the binding of a hormone to a cell surface receptor). A principal result of the activation of PKA, via intracellular production of cAMP, is the breakdown of glycogen in the liver and subsequent transport of glucose to the bloodstream, maintaining blood glucose levels between meals.

Right: The structure of the catalytic subunit of PKA (figure generated from PDB file 1atp using PyMOL). The main-chain fold, shown as a cyan ribbon, has a two-lobed structure with a cleft between them where substrate ATP (shown in stick form) binds. A 20-residue peptide that includes a pseudosubstrate sequence is also bound in the active site cleft (shown in yellow), forming a ternary complex. The pseudosubstrate peptide forms part of the regulatory subunit of PKA holoenzyme.