BIOCHEMISTRY TOPICS
Lipids: Structures and properties
Defining lipids. Classification of lipids. Properties of lipids and relevance to biological membranes, and biosignaling.
Lipids (colloquially known as "fats") are a class of molecules - largely (but not exclusively) derived from biological sources - that have a significant nonpolar character. Thus, one criterion for a lipid is solubility in nonpolar solvents. However, most lipids are not completely nonpolar, but include at least a small polar or charged component or functional group. This gives them a more or less dual nature - both nonpolar and polar - a characteristic termed amphipathic.
Fats, fatty acids, soaps.
Fatty acids are carboxylic acids, with the functional group at one end of a rather long, unbranched hydrocarbon chain. Fatty acids are thus amphipathic - the carboxylic acid is ionized at physiological pH (making it a negatively-charged carboxylate group) and interacts well with a polar solvent (the carboxylate group is hydrophilic, or water-loving), while the hydrocarbon chain is quite nonpolar, and contributes a hydrophobic effect.
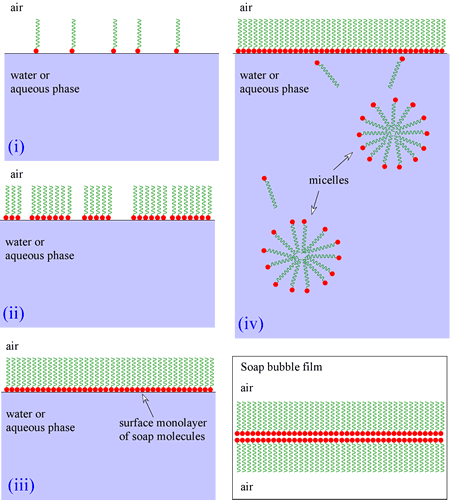
The behavior of lipid molecules forced within an aqueous environment is quite interesting. For example, the salts of fatty acids, which are classified as soaps (for example, sodium stearate), at first migrate to the surface to begin to form a monolayer, with the nonpolar "tails" projecting into the air. (Air is a more hydrophobic environment, especially compared to water.) When the surface becomes completely covered with soap molecules, micelles will begin to form within the liquid phase. The formation of micelles by soaps and detergents occurs spontaneously in aqueous media when the concentration of soap reaches this threshhold, which is called the critical micelle concentration (CMC). The principle that relates these behaviors is the tendency for nonpolar regions to clump together, seqestering these regions as much as possible from the aqueous environment. The carboxylate groups (and associated ions) orient to interact with the aqueous phase. When one shakes up a soap solution, soap bubbles form - something that can be explained by the same relatively simple physiochemical principle summarized by the phrase "like interacts with like". The thin film of a soap bubble is actually a bilayer with the nonpolar regions on the surfaces (both inside and outside the bubble), and the polar/charged portions in this case sequestered (or "sandwiched") in the middle.
Triacylglycerols
Triacylglycerols (also called triacylglycerides or triglycerides) are a common, simple type of lipid consisting of three long-chain fatty acids esterified to glycerol, a three-carbon triol. They are the typical storage form of fats in adipose tissue, providing about 9 kcal/g of energy. (Compare with 4 kcal/g provided by carbohydrates and proteins.)

The figure at right shows a typical triacylglycerol as a structural (bond-line) formula. Carbon atoms are indicated at vertices and ends of lines. Hydrogen atoms are implicit. The three-carbon glycerol backbone is drawn in black. Three fatty acids (green, red, blue) are shown in ester linkages with glycerol.
The green fatty acid chain is from palmitate, a 16-carbon saturated fatty acid. Triacylgycerols are mostly carbon and hydrogen, giving them a predominently nonpolar character. The ester linkages of the molecule give it a somewhat polar end.
Phospholipids and membranes
In contrast to the soaps discussed above, other lipids common in biological membranes have a larger van der Waals cross section and cannot approach one another close enough to form micelles. Instead, they spontaneously form a lipid bilayer.
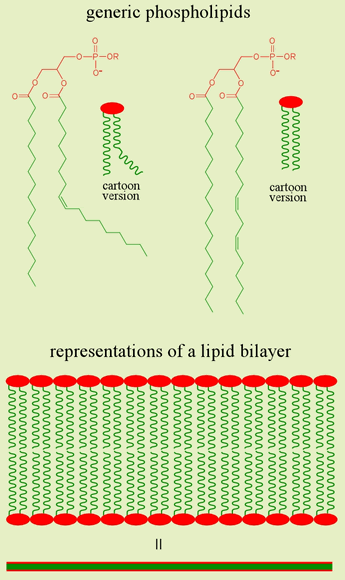
At right is shown the structural formula and cartoon versions of generic membrane phospholipids. A membrane phospholipid typically consists of a glycerol backbone, two fatty acid chains in ester linkages to glycerol, a phosphate diester linkage between glycerol and a number of possible alcohols (ROH). It is the presence of two nonpolar fatty acid chains in phospholipids (in contrast to the single chain of soaps) that favors bilayer over micelle formation for steric reasons. The first fatty acid is of the saturated variety, while the second is typically an unsaturated fatty acid chain. The cis configuration of the latter confers a rigid kink in the chain, thus the first phospholipid cartoon is more stereochemically accurate, but the second cartoon is more suited to cartoon versions of bilayers and biological membranes.
The existence of cells is obviously dependent on creation of a boundary that defines an inside compartment of controlled composition and character, and separates the cell from the surrounding uncontrolled environment. Cellular membranes are topologically closed surfaces based upon phospholipid bilayers that perform this bounding function. Biological membranes act as physical barriers that generally limit the passage of charged and polar species, as well as the macromolecules central to living systems. The amphipathic nature of phospholipids is responsible for the spontaneous formation of the bilayer structure of membranes.
In the schematic representation of a lipid bilayer shown above, the amphipathic nature of the constituent phospholipids is indicated by the red spheres symbolizing the charged/polar "head" groups, and the long hydrocarbon "tails" (green lines). In aqueous media, these molecules assemble into a bilayer structure with the tails sequestered in a nonpolar interior, and heads interacting with polar solvent and solutes.
Note that the thermodynamic basis for spontaneous assembly of lipid bilayers (ΔG < 0) is another example of the significance of the hydrophobic effect.
Classification of biological membrane lipids
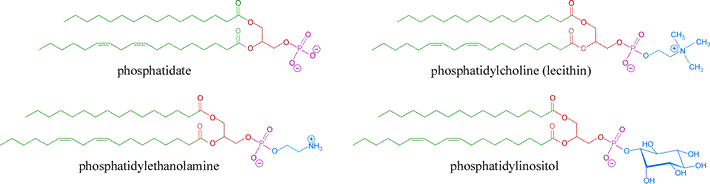
There are many different lipids found in biological membranes, helping account for the different properties and functional roles membranes play in cellular biology. The phospholipids are the largest proportion in most membranes, and consist of two types, the glycerophospholipids (introduced above) and the phosphocholine sphingolipids (sphingomyelin). The glycerophospholipids can be considered derivatives of phosphatidate (or phosphatidic acid) in which it combines with other alcohols to form phosphatidyl esters, generating the variety of membrane lipids of this class. Some important membrane glycerophospholipids are illustrated in the figure below.
Sphinglipids are sphingosine-containing lipids, but are either phospholipids or glycolipids, depending on whether a phosphoester or a glycan (mono- or oligosaccharide) is attached to ceramide. The next figure illustrares the structure and the component composition of sphingomyelin.
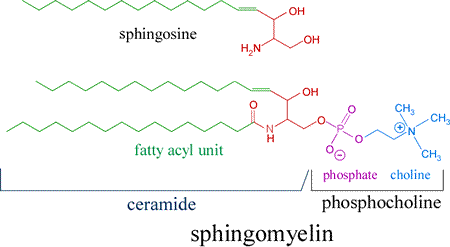
Note the resemblance to glycerophospholipids - in effect, the amphipathic amino alcohol sphingosine repaces the glycerol backbone and one of the fatty acid chains of glycerophospholipids.
Below is shown a symbolic representation of common membrane phospholipids and their components. For example, when the alcohol is the quaternary amine alcohol choline, the result is a phosphatidylcholine, which has the common name lecithin.
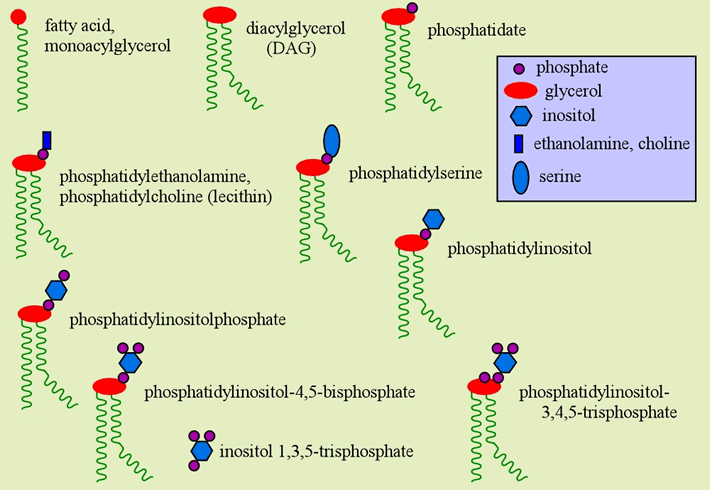
Inositol 1,3,5-trisphosphate is not a lipid, but is a constituent of the phosphoinositides. The latter subclass of membrane phospholipids provide an example of the role of lipid constituents in signal transduction pathways. Inositol 1,3,5-trisphosphate (IP3) and diacylglycerol (DAG) are generated from phosphatidylinositol-4,5-bisphosphate being acted on by a (class C) phospholipase. IP3 acts as a so-called "second messenger" that mobilizes calcium ion (Ca2+) from storage in the endoplasmic reticulum, while DAG remains in the membrane and activates protein kinase C.
Cholesterol
Cholesterol is another type of biologically important lipid. Its structure is distinct in that a large portion of it is made up of four joined rings. This system of rings, characteristic of steroids, is not only nonpolar, but it is structurally quite rigid - a direct consequence of the rings.
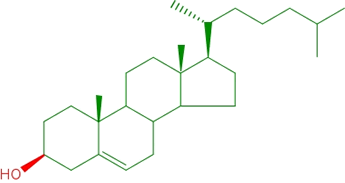
The figure at left illustrates the structural formula for cholesterol. The polar part of the molecule consists of a single hydroxyl group. Cholesterol is found principally in the cellular membranes of animals, which is why animal fat is considered high cholesterol, while plant sources are much lower in cholesterol content. The rigidity of the rings of the steroid nucleus has profound effects on how the properties of membranes (such as fluidity and temperature range over which a melting transition occurs) vary with varying cholesterol content.
Cholesterol, and other steroids (which are familiar to many as hormones and performance-enhancing substances) are examples of a broad class of compounds called isoprenoids.
Related topics pages: