BIOCHEMISTRY TOPICS
Carbonic anhydrase
Catalysis with a metal ion cofactor.
Carbonic anhydrase [EC 4.2.1.1] is a zinc metalloenzyme that catalyzes the interconversion of carbon dioxide and bicarbonate ion by hydration/dehydration.
Carbonic anhydrase (CA) was the first enzyme to have been discovered to contain zinc and is still one of the prototypes of zinc-containing enzymes. The CA isozymes from mammals are among the most well-studied enzymes in biochemistry. Since the interconversion reaction occurs at a significant uncatalyzed rate in solution, the rate enhancement by CA is modest. Nonetheless, some CAs are among the fastest enzymes known. The human isozyme CA II has a kcat/KM of 1.5 × 108 M−1sec−1, a value for catalytic efficiency that approaches the theoretical limit of diffusion control.
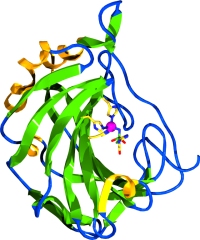
The figure at left shows the structure of a human CA isozyme. The ribbon representation of protein secondary structure shows that it is mostly a β sheet. The twist of the beta sheet creates an active site. pocket, at the bottom of which lies the catalytic zinc, shown in the figure as a magenta sphere.
The side chains of the histidine residues that coordinate the zinc ion are shown, as well as an an inhibitor that occupies the fourth coordinate position. The role of zinc in CA is that of an electrophilic catalyst. The pKa of zinc-coordinated water is lowered to such an extent that a significant proportion exist as nucleophilic (and reactive) hydroxide ion at physiological pH.
Recall that CO2, as a metabolic product, is an acid anhydride, meaning it reacts with water to produce carbonic acid, which in turn is an unstable species that rapidly decomposes producing hydronium ion (usually we'll abbreviate this as H+(aq)) and bicarbonate.
Zinc is the key
Zinc is an essential element for eukaryotic and probably most organisms, and it is the second most abundant trace metal in most higher animals (after iron). Its role as a key component of numerous enzymes, carbonic anhydrase being a prominent example. Others include alcohol dehyrogenase, zinc metalloproteases such as thermolysin and carboxypeptidase A, and class II aldolase from bacteria. The occurrence of zinc in an impressive variety of proteins makes the biochemistry of zinc a fascinating and diverse specialty. The properties of zinc that determine its biological utility include a flexible coordination geometry, Lewis acidity, intermediate polarizability, and redox stability. In proteins, zinc is most commonly observed in a tetrahedral coordination geometry, with the side chains of His, Cys, and Asp or Glu as ligands. In zinc enzymes, water is often a ligand, in which case the ability of zinc to act as a Lewis base is often the key to the catalytic mechanism. The pKa of zinc-coordinated water is lowered to such an extent that a significant proportion exist as nucleophilic (and reactive) hydroxide ion at physiological pH.
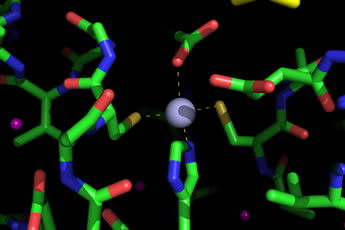
Left: The zinc ion at the catalytic center, or active site, of carbonic anhydrase from pea chloroplast. The zinc ion (light blue) is bound by a histidine side chain from below via its Nε2 atom, and two cysteine side chains on either side. A roughly tetrahedral coordination sphere is completed by an oxygen from acetic acid (above), here acting as an analog of the substrate bicarbonate. Drawn from pdb file 1ekj (see Kimber & Pai, 2000), using PyMOL.
Carbonic anhydrases and convergent evolution
Carbonic anhydrases provide a striking case of enzymes of completely different overall structure and phylogenetic origin having arisen independently, yet adopting the same basic catalytic strategy. By far the best characterized are the α (alpha) class CAs, often represented in biochemistry textbooks by human CA II (shown above in the first structure figure), a CA isozyme that is plentiful in erythrocytes (red blood ncells). The β (beta) class is the next most well-studied, and the β-CAs are important in higher plants and many prokaryoic and simple eukaryotic organisms, both photosynthetic and heterotrophic. The following key points relate to convergent evolution of the CAs.
- There are three main phylogenetically and structurally distinct CA classes: α, β, γ.
- The α class is mainly mammalian and is thought to have originated ~ 600 Mya
- The β and γ classes are widely distributed
- Both the β and γ classes are thought to be ancient enzymes, originating from around the time of divergence of the three major domains of life. The γ class in particular is thought to be at least 4.2 billion years old!
Below shows representative structures of β and γ CAs: (left) E. coli carbonic anhydrase (ECCA), a β-CA; and (right) an archaeal γ-CA from Methanosarcina thermophila. The β-CA is the only CA class with significant α helix content.
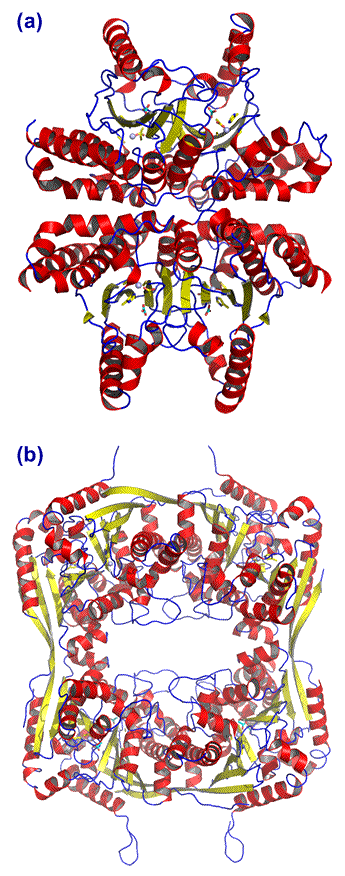
Above: Structure of γ-CA from Methanosarcina thermophila. (a) Ribbon diagram of the trimeric gamma carbonic anhydrase. (b) Same as (a), but rotated about 90° around a horizontal axis in the plane of the figure. The γ-CA monomer displays an unusual "beta helix" architetcture. Drawn from pdb file 1qrg (see Ref. 7).
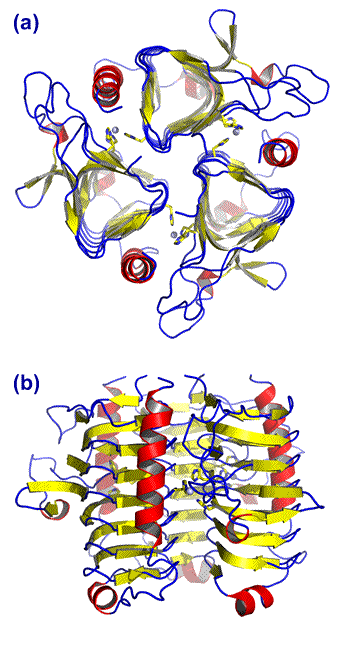
Above: Two representative β-CA structures. (a) E. coli carbonic anhydrase (ECCA). Drawn from pdb file 2esf (see Ref. 5). (b) Octomeric plant CA, from Pisum sativum (garden pea) chloroplast. Drawn from pdb file 1ekj (see Ref. 6).
The ribbon diagrams (above and left) show α helices in red, β strands in yellow, and connecting loops in blue. The zinc ions are represented as small gray spheres, and ligands to the metal are shown explicitly in stick form.
Related topics pages: