Lecture 4. Buffers and their biological roles
Thursday 24 January 2019
Buffers as balanced mixtures of a weak acid and its conjugate base. The Henderson-Hasselbalch equation. Examples of buffer preparation calculations. Phosphate species and their pKa values. Carbon dioxide and the bicarbonate buffering system.
Reading: Lehninger - Ch.2, pp.63-69.
Summary
Reading summary.
§2.3 Buffering against pH changes in biological systems.
Buffers are mixtures of weak acids and their conjugate bases.
Weak acids or bases buffer cells and tissues against pH changes.
Worked example 2-5: Ionization of histidine.
Worked example 2-6: Phosphate buffers.
Untreated diabetes produces life-threatening acidosis.
Box 2-1 -Medicine- On being one's own rabbit (don't try this at home!).
Worked example 2-7: Treatment of acidosis with bicarbonate.
§2.4 Water as a reactant.
§2.5 The fitness of the aqueous environment for living organisms.
***
Starting with the chemical equation for the ionization of a weak acid (see figure below, left), the acid dissociation equation (top equation), we derive the usual equilibrium expression, giving the equilibrium constant the special designation Ka (2nd equation). Taking the negative logarithm (base 10) of both sides of this equation leads to a definition of pKa in terms of pH and the log(10) of the ratio of concentrations of the anionic conjugate base and unionized acid (not shown).
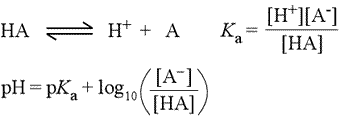
This equation can be rearranged to express pH in terms of pKa and the log ratio of conjugate base to acid, yielding what is known as the Henderson-Hasselbalch equation (3rd equation, at left).
These equations are very useful for buffer calculations and in determining the proportions of conjugate pairs that exist at a given pH. It is easy to see, for instance, that at a pH equal to the pKa of a weak acid HA, the population of unionized acid molecules is exactly balanced by the number of molecules of the conjugate base. At a pH above that of the pKa, the anionic form will predominate, and at low pH, the unionized acid species is favored.
Exercise: Derive the Henderson-Hasselbalch equation for the ionization equilibrium BH+ = H+ + B:. Which form predominates at low pH? Compare this equilibrium with that for the dissociation of the neutral acid HA above, and consider the effect of transferring the ionizable group from an aqueous environment to a non-polar environment. Are the respective pKa's affected equally, or is one affected more than the other? Explain.
Phosphate

One of the most important inorganic ions in biochemistry, phosphate plays a central role in the energetics of metabolism, biological structure, and regulation. Inorganic phosphate (often abbreviated Pi, also sometimes called orthophosphate) in aqueous solution exists primarily as H2PO4- or HPO4-2, and is an effective buffer at physiological pH (7.0 - 7.5). Phosphate forms esters with the hydroxyl groups of a variety of biomolecules of all sizes, notably sugars and proteins
Phosphate, with its four distinct protonation/charge states, constitutes a triprotic system. The fully protonated form of phosphate is phosphoric acid. Strictly speaking, the term "phosphate" refers to the fully deprotonated form. Neither of these forms exists in any significant quantity at or near physiological pH. The figure below illustrates all four of the species (in full Lewis structure representations, showing nonbonding electrons as well as covalent bonds). The predominant forms between pH 4 and 10 are the two intermediates, dihydrogen phosphate (monobasic) and monohydrogen phosphate (dibasic).
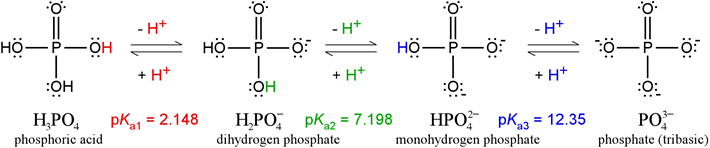
The Henderson-Hasselbalch equation can be applied to tell us the proportions of dihydrogen phosphate and monohydrogen phosphate present in a buffered system between pH 4 and 10. For instance, at pH = pKa of dihydrogen phosphate, the amounts of dihydrogen and monohydrogen forms are exactly the same. The concentrations of phosphoric acid and tribasic phosphate are well below negligible, and the average charge of phosphate species in solution is –1.5.
Carbon dioxide and bicarbonate
Carbon dioxide acts chemically in aqueous solution as an acid anhydride (anhydride means "without water"), meaning it reacts with water, to form carbonic acid. The conjugate base of carbonic acid is bicarbonate, an intermediate species buffering agent. The figure below depicts the equilibria involved, along with the pKa values. The rapid enzymatic conversion of carbon dioxide to bicarbonate, and its reverse, is also indicated.
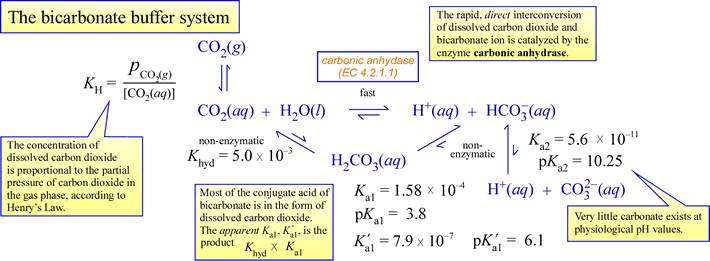
A few important points to tie all the chemistry above to physiology:
- A biological system is open, meaning it exchanges matter and energy with its surroundings. In this scenario, this means (for example) that excess metabolic carbon dioxide is expelled to the surroundings.
- Carbon dioxide is a neutral, nonpolar molecule, and can readily diffuse across membranes. Bicarbonate is a charged species, and does not cross membranes at a significant rate unless facilitated by transmembrane channels.